Recent Advance in Acute Lymphoblastic Leukaemia
Volume 14, Issue 2, August 2019 (download full article in pdf)
Editorial note
In this topical update, Dr Albert Sin reviews recent advances in diagnosis and management of acute lymphoblastic leukaemia (ALL). We welcome any feedback or suggestions. Please direct them to Dr Rock Leung (e-mail: leungyyr.ha.org.hk) of Education Committee, the Hong Kong College of Pathologists. Opinions expressed are those of the authors or named individuals and are not necessarily those of the Hong Kong College of Pathologists.
Dr. Albert Sin
Clinical Assistant Professor
Introduction
Acute lymphoblastic leukaemia (ALL) is an aggressive and highly fatal malignancy resulting from clonal mutations of lymphoid progenitor cells. The incidence of ALL is the most common in childhood and age after 50.18The prognosis of childhood ALL is good with long-term survival rate approaching 90% treated by intensive chemotherapy.12Although the incidence of ALL is less in adolescent, young adult as well as adult, the prognosis of ALL in those people is very poor, with only 30-40% of adult patients able to remit.18 According to the data from US database which registered all patients with diagnosed ALL from 2000 to 2007, the survival rate was 75% at 17 years old, 45% at 20 years old and 15% at 70 years old.14An increasing knowledge of disease biology of ALL transformed into insights for development of novel therapies to improve the treatment outcome of ALL.
One of the reasons of adverse prognosis in adolescent and young adult (AYA) as well as adult patients is that they commonly harbored poor-risk genetic aberrations while less patients carried favorable genetic lesion.14This could explain the sudden drop in survival from 17 years old to 20 years old.
Ph-like ALL
Ph-like ALL is a newly identified genetic subgroup. The genetic profile of this subgroup of ALL is similar to that of Philadelphia chromosome positive (Ph-positive) ALL but without BCR-ABL1 fusion.17They have a higher frequency of IKZF1 deletion and mutation in genes of lymphoid transcription factors with poor survival.19The incidence of Ph-like ALL increases with ages and approaching 27% of cases of adult B-ALL.14
The nature of genetic aberration is heterogeneous. Despite its complexity, it can be simply classified into five subgroups: 1. CRLF2 rearrangement 2. Rearrangement of ABL-class gene 3. Rearrangement of JAK2 and EPOR 4. Aberrations leading to activation of JAK-STAT or MAPK pathway 5. Other rare kinase alterations.19The distribution of different types of genetic alterations are different among childhood high risk ALL, young adult and adult (Figure 1). CRLF2 rearrangement is the most common type of genetic alteration in Ph-like ALL. CRLF2 gene is responsible for producing lymphopoietin receptor and regulate the process of lymphopoiesis. Common mechanisms of CRLF2 rearrangement include 1. Translocation of CRLF2 gene into IGH gene 2. Fusion between CRLF2 gene and P2RY8 gene. 3. Point mutation F232C at CRLF2 gene. Nearly 50% of CRLF2 rearranged Ph-like ALL have concomitant JAK mutations.19
Figure 1
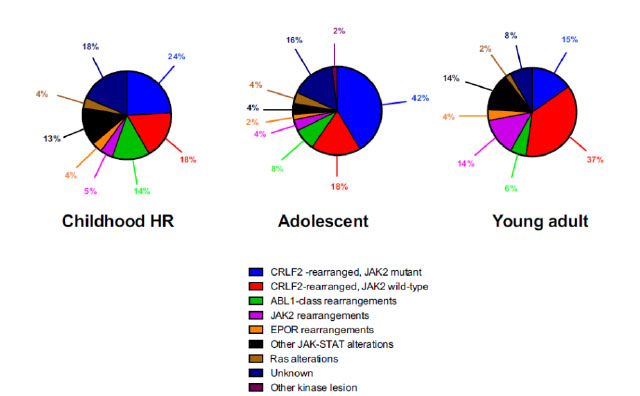
Diagnosis of Ph-like ALL
Genetic profiling is the gold standard for the diagnosis of Ph-like ALL. However, it is difficult to implement in routine diagnostic laboratory.
Cytogenetics analysis is a standard test for all cases of ALL which allows a global assessment of chromosomal abnormalities. Some of the recurrent genetic abnormalities, for example t(9;22), hyperdiploidy/hyperdiploidy, rearrangement involving 11q23, etc, can be detected. However, most of the Ph-like ALL genetic alterations are cryptic, e.g. interstitial deletion of CRLF2, ETV6-RUNX1 fusion, etc and thus they cannot be detected by conventional cytogenetics.2
Fluorescent in-situ hybridization (FISH) can be utilized to detect Ph-like ALL genetic abnormalities. Breakapart probes targeting genes most frequently genes including ABL1, ABL2, PDGFRB, JAK2, CRLF2, and P2RY8 are currently available. Although the positive result upon FISH study needs additional fusion probe for confirmation, it provides a readily available and useful diagnostic tool for establishing the diagnosis of Ph-like ALL. However, some of the important Ph-like ALL genetic rearrangement including intrachromosomal inversions (e.g., inv(9) resulting in PAX5-JAK2 fusion), intra-chromosomal deletions (e.g., del(X)(p22p22)/del(Y)(p11p11) resulting in P2RY8-CRLF2 fusion) are undetectable by FISH technique.2Targeted sequencing by NGS platform is an evolving technique for diagnosis.16
Recently, antibody against CRLF2 is available for flow cytometry study. The expression of CRLF2 as detected by multiparametric flow cytometry is correlated with genetic testing for CRLF2 rearrangement.15This provides a rapid tool for identifying potential cases of Ph-like ALL before the result of genetic tests is available.
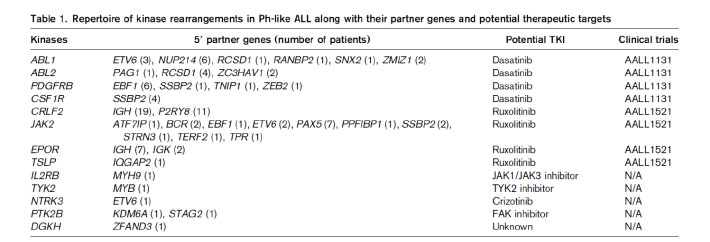
Most of the genetic alterations of Ph-like ALL are targetable kinase lesions, which could be treated by tailored kinase inhibitor therapy (Table 1).19This approach of therapy is current undergoing extensive preclinical studies.9
Early T-cell precursor ALL (ETP-ALL)
ETP-ALL is recently characterized subtype of T-ALL. It constitutes around 12% of childhood ALL and 7.4% of adult ALL.11Genetic profiling showed ETP cells are similar to that of haemopoietic stem cells and myeloid progenitor cells.4This subgroup of ALL is characterized by the unique immunophenotype: cytoplasmic CD3+, surface CD3-, CD1a-, CD2-, CD5 dim (<75% positive), CD7 and positive for one or more stem cell and/or myeloid markers including HLA-DR, CD13, CD33, CD34, or CD117.5
While activating mutation of NOTCH1 is a common mutation found in ALL and it account for 50% of cases of childhood ALL, this mutation is less common in ETP-ALL.3ETP-ALL commonly have mutations in FLT3, DNMT3A, IDH1, IDH2, etc.11
ETP-ALL carries a poor prognosis with inferior overall survival when treated with standard chemotherapy regimen comparing with other subtypes of T-ALL.11This subgroup of T-ALL represented a distinct subtype with unique genetic profile and poor prognosis.
MRD in adult ALL
Minimal residual disease (MRD) describe the very low level of disease burden which cannot be detected by morphology. Measurement of MRD not only pick up a submicroscopic level of disease but also can monitor the disease kinetic during the treatment process of haematological malignancies.10
The following techniques can be used to detect MRD:
- Multiparametric flow cytometry to detect leukaemia-associated immunophenotype (LAIP)
By using a 4-color or 6-color panel of antibodies, we can identify LAIP in 90% of ALL caes.10Flow cytometry is a quick method and the result of MRD can be generated in a short period of time for clinical decision. The sensitivity of MRD detection by this method is 0.01%. However, in order to define the positive MRD, we need 10-40 cluster of cells and thus higher number of cells are required for assessment which may be difficult for reassessment samples after intensive chemotherapy.7In addition, antigenic shift is commonly occurred in leukaemic cells and normal cells during the therapy. The use of monoclonal antibodies, e.g anti-CD19, anti-CD22 for treatment of ALL will affect the gating strategy used to identify the leukaemic cells.10 - Detecting leukaemia-specific fusion transcript by PCR technique
Quantitative reverse-transcriptase PCR can be employed to detect the amount of leukaemia-specific fusion transcript. The sensitivity is higher compared with flow cytometry (10-4to 10-6).7The test is relatively easy to be performed in standardized diagnostic laboratory. However, only 30-40% of cases of ALL carry leukaemia-specific fusion transcript and thus limited the eligibility of MRD detection by this method. Moreover, the interpretation is challenging for RNA-based test in those cases will poor RNA quality. - Quantitative PCR for immunoglobulin (IG)-T cell receptor (TCR) gene targets
Quantitative PCR is employed to detect the specific sequence of rearranged IG gene or TCR gene in the sample. The sensitivity of this method is 10-4to 10-5and this method can be applied to all cases of ALL. However, this method of MRD detection requires prior characterization of IG or TCR gene rearrangement by sequencing and designs patient-specific primers for each case for subsequent MRD detection. Extensive standardization and experience are needed for the laboratory to set up this test, which limit the use of this method of MRD detection in diagnostic laboratory. Moreover, the clonal evolution in leukaemic blasts during treatment can make the original rearranged sequence to be lost and thus generate a false negative result. Also, the non-specific primer annealing occurs during the process of marrow regenerative may yield false positive result for the test.7
Application of MRD in treatment of adult ALL
MRD-guided therapy had been gained extensive experience in childhood ALL.8The study group of German Multicenter Study Group for Adult ALL (GMALL) had conducted largest study for the role of MRD in adult Ph-negative ALL. They showed that molecular remission is the only parameters significantly affect the remission duration and survival.6Patients with positive MRD after induction therapy achieved better overall survival after receiving haemopoietic stem cell transplant. Early achievement of MRD negativity after induction chemotherapy is associated with good outcome for adult ALL.10Study showed that MRD level correlates with post-transplant outcome.13Another group found that haemopoietic stem cell transplant benefits the patients with positive MRD at week 6.1These findings may prompt reconsideration of the indications of haemopoietic stem cell transplant for adult patients with ALL, especially those patients achieve MRD negativity after treatment.
Concluding landmark
The prognosis of acute lymphoblastic leukaemia in young adolescent and adult is poor. The recent discovery of new subtype of acute lymphoblastic leukaemia with characterization of genetic lesions make a breakthrough of understanding of disease biology. Precise disease prognostication can be made. Targeted therapies are being developed for treating those patients. Clinical trials are conducting for evaluating the targeted therapies in those new subtypes of acute lymphoblastic leukaemia. Moreover, the application of MRD monitoring and MRD-adapted therapy in adult ALL can further stratified the patients and select the appropriate candidates of haemopoietic stem cell transplant in order to reduce transplant-related mortality and morbidity. The advances in understanding of molecular mechanism and disease biology of ALL help to improve the risk stratification, rapid development of targeted therapies and hopefully improve the prognosis in young adolescent and adult patients.
Reference
- Beldjord K., Chevret S., Asnafi V., Huguet F., Boulland ML., Leguay T., Thomas X., Cayuela JM., Grardel N., Chalandon Y., Boissel N., Schaefer B., Delabesse E., Cavé H., Chevallier P., Buzyn A., Fest T., Reman O., Vernant JP., Lhéritier V., Béné MC., Lafage M., Macintyre E., Ifrah N., Dombret H; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). (2014) Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood, 2014,12;123(24):3739-49.
- Bradford J. Siegele., Valentina Nardi. (2018). Laboratory testing in BCR-ABL1-like (Philadelphia-like) B-lymphoblastic leukemia/lymphoma. Am J Hematol, 2018(93):971–977.
- Chao Gao., Shu-Guang Liu., Rui-Dong Zhang., Wei-Jing Li., Xiao-Xi Zhao., Lei Cui., Min-Yuan Wu., Hu-Yong Zheng and Zhi-Gang Li. (2014) NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol, 2014, 166(2):221-8. doi: 10.1111/bjh.12866
- Elaine Coustan-Smith., Charles G Mullighan., Mihaela Onciu., Frederick G Behm., Susana C Raimondi., Deqing Pei., Cheng Cheng., Xiaoping Su., Jeff rey E Rubnitz., Giuseppe Basso., Andrea Biondi., Ching-Hon Pui., James R Downing., Dario Campana. (2009) Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia.Lancet Oncol, 2009(10):147–56
- Elizabeth A. Raetz and David T. Teachey. (2016) T-cell acute lymphoblastic leukemia. Am Soc Haemaetolo Educ Program, 2016(1), 580-588
- Gökbuget N., Kneba M., Raff T., Trautmann H., Bartram CR., Arnold R., Fietkau R., Freund M., Ganser A., Ludwig WD., Maschmeyer G., Rieder H., Schwartz S., Serve H., Thiel E., Brüggemann M., Hoelzer D; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. (2012) Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood, 2012,120(9):1868-76.
- Jacques J. M. van Dongen., Vincent H. J. van der Velden., Monika Br¨uggemann and Alberto Orfao. (2015) Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies.Blood, 2015;125(26):3996-4009
- Marianne Ifversen., Dominik Turkiewicz., Hanne V. Marquart., Jacek Winiarski., Jochen Buechner., Karin Mellgren., Johan Arvidson., Jelena Rascon., Lenne-Triin Ko¨rgvee., Hans O. Madsen., Jonas Abrahamsson., Bendik Lund., Olafur G. Jonsson., Carsten Heilmann., Mats Heyman., Kjeld Schmiegelow., Kim Vettenranta. (2019) Low burden of minimal residual disease prior to transplantation in children with very high risk acute lymphoblastic leukaemia: The NOPHO ALL2008 experience. British Journal of Haematology, 2019(184): 982–993
- Maude SL, Tasian SK, Vincent T, et al. (2012) Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood, 2012;120(17):3510-3518.
- Monika Bru¨ ggemann and Michaela Kotrova. (2017) Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Hematology Am Soc Hematol Educ Program.2017(1):13-21. doi: 10.1182/asheducation-2017.1.13.
- Nitin Jain., Audrey V. Lamb., Susan O’Brien., Farhad Ravandi., Marina Konopleva., Elias Jabbour., Zhuang Zuo., Jeffrey Jorgensen., Pei Lin., Sherry Pierce., Deborah Thomas., Michael Rytting., Gautam Borthakur., Tapan Kadia., Jorge Cortes., Hagop M. Kantarjian., Joseph D. Khoury. (2016) Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood, 2016;127(15):1863-1869
- Paul, S., Kantarjian, H., & Jabbour, E. J. (2016). Adult Acute Lymphoblastic Leukemia. Mayo Clin Proc, 91(11), 1645-1666. doi:10.1016/j.mayocp.2016.09.010
- Renato Bassan., Monika Brüggemann., Hoihen Radcliffe., Elizabeth Hartfield., Georg Kreuzbauer., Sally Wetten. (2019) A Systematic Literature Review And Meta-Analysis Of Minimal Residual Disease As A Prognostic Indicator In Adult B-Cell Acute Lymphoblastic Leukemia. Haematologica,2019 Mar 19. pii: haematol.2018.201053. doi: 0.3324/haematol.2018.201053.
- Roberts KG. (2018) Genetics and prognosis of ALL in children vs adults. Hematology Am Soc Hematol Educ Program. 2018(1):137-145. doi: 10.1182/asheducation-2018.1.137.
- Sergej Konoplev., Xinyan Lu., Marina Konopleva., Nitin Jain., Juan Ouyang., Maitrayee Goswami., Kathryn G. Roberts., Marc Valentine., Charles G. Mullighan., Carlos Bueso-Ramos., Patrick A. Zweidler-McKay., Jeffrey L. Jorgensen and Sa A. Wang. (2017) CRLF2-Positive B-Cell Acute Lymphoblastic Leukemia in Adult Patients: A Single-Institution Experience. Am J Clin Pathol, 2017(147):357-363
- Stadt UZ., Escherich G., Indenbirken D., Alawi M., Adao M., Horstmann MA. (2016) Rapid Capture Next-Generation Sequencing in Clinical Diagnostics of Kinase Pathway Aberrations in B-Cell Precursor ALL. Pediatr Blood Cancer, 2016;63(7):1283-6. doi: 10.1002/pbc.25975
- Tasian, S. K., Loh, M. L., & Hunger, S. P. (2017). Philadelphia chromosome-like acute lymphoblastic leukemia. Blood, 130(19), 2064-2072. doi:10.1182/blood-2017-06-743252
- Terwilliger, T., & Abdul-Hay, M. (2017). Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J, 7(6), e577. doi:10.1038/bcj.2017.53
- Thai Hoa Tran and Mignon L. Loh. (2016). Ph-like acute lymphoblastic leukaemia. Haematology Am Soc Haemaetolo Educ Program, 2016(1), 561-566.
